Introduction
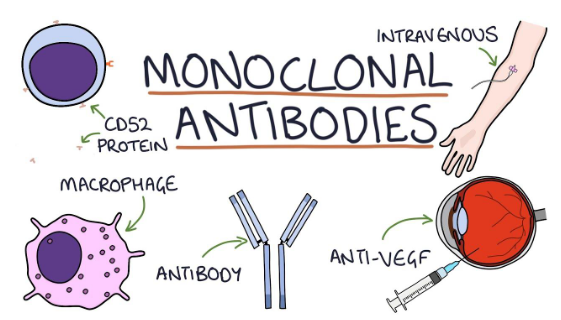
Monoclonal antibodies(mAb or moAb) are bio-engineered proteins that are used as human antibody fill-ins. They can modify, enhance, mimic or restore an individual’s immune system to attack foreign substances. Monoclonal antibodies have the ability to identify an epitope(binding site) of a single antigen or sometimes a precise binding site of a cancerous cell.
Discovery of monoclonal antibodies
Monoclonal antibodies were discovered in 1975 by prominent scientists Georges Kohler and Cesar Milstein. They synthesized the monoclonal antibodies(mAb or moAb) by fusing murine-myeloma cells with lymphocytes that secrete murine-antibodies. However, monoclonal antibodies prepared from murine may trigger an immunogenic reaction in human patients that reduces the effectiveness of immunotherapy. As a possible course of action, humanized and chimeric antibodies were developed that unlikely stimulate the immune response in human patients.
How are monoclonal antibodies(mAb or moAb) produced?
The production of Monoclonal antibodies(mAb) requires clones or cell lines from immunized animals. The cell culture is prepared by fusing myeloma cells with the B-cells obtained from the immunized animals as described by Kohler and Milstein.
The conventional procedure for the production of monoclonal antibodies is the hybridoma technology wherein the murine-myeloma cells are fused with the spleen cells from immunized mice. Myeloma cells immortalize the hybridoma cells which serves the purpose of indefinite cultivation of cell culture, and spleen cells(B-cells) grant antigen specificity. In view of the fact that each hybridoma is obtained from a single cell and the hybridoma cell culture is a homogeneous mixture, it makes identical copies of antibodies with the same antigen-binding sites and isotype, as such, it is called a monoclonal antibody.
The standard method for the production of monoclonal antibodies (mAb or moAb) is hybridoma technology.
For producing monoclonal antibodies at a large-scale, ascites tumours are generated in mice or in vitro mammalian cell culture fermentation with the help of continuous perfusion culture systems and bioreactors. Large-scale production involves major issues like growth media, fermentation time, size of the fermenter, and the process of purification.
The purification process also known as the downstreaming process can be achieved by fragmentation, chromatography, ultrafiltration, controlled precipitation, and conjugation with chelating agents.
Working principle of monoclonal antibodies(mAbs)
Monoclonal antibodies work in various ways that depend on the type of protein they target. Some monoclonal antibodies also serve the purpose of immunotherapy by stimulating the immune response to cancer cells.
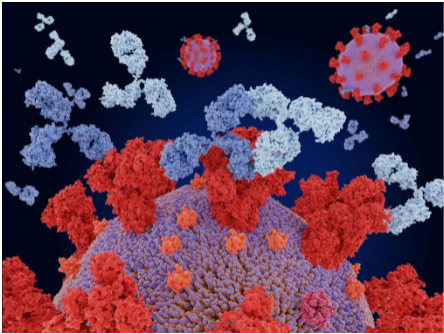
Monoclonal antibodies deactivate coronavirus by attaching themselves to it thereby helping the immune system to locate the virus.
They trigger the immune system in one or more ways;
- to attack and kill the cancer cells
- and/or they get attached to cancer cells and help the immune system in locating them- this process is known as antibody-dependent cell-mediated cytotoxicity or ADCC.
- Monoclonal antibodies that show ADCC working principle are rituximab(Mabthera), cetuximab(Erbitux) and trastuzumab(Herceptin).
PD-1 and PD-L1 inhibitors suppress the activity of check point proteins that stop the immune system from attacking the cancer cells.
- Some mAbs work as immunotherapy. They act on cells of the immune system such as checkpoint inhibitors- (ipilimumab, nivolumab).
- These checkpoint inhibitors block proteins like PD-1 inhibitors, PD-L1 inhibitors that in turn halts the action of the immune system attacking the cancer cells.
- Other mAbs block the protein-cell interactions required for the growth of new blood vessels, thereby stopping the blood supply.
Side effects and applications of monoclonal antibodies
As mAbs are proteins, their application may sometimes cause an allergic response like fever, weakness, headache, nausea, diarrhoea, chills, rashes, low blood pressure; and consequential infections like heart attack, high blood pressure, congestive heart failure, inflammatory lung diseases etc., to name a few.
Monoclonal antibodies are used as IV injections for cancer treatment.
Monoclonal antibodies(mAb or moAb) are given in the form of IV injections; that is intravenously injected straightaway into the bloodstream via veins.
Monoclonal antibody therapy has not been sufficiently studied in pregnant and/or in breastfeeding women. Monoclonal antibodies like pembrolizumab(Keytruda) and nivolumab(Opdivo) are considered to have harmful effects on the foetus. Breastfeeding women are advised to discontinue the usage of monoclonal antibodies as they may be released in breast milk and could eventually have dreadful effects in infants.
The employment of monoclonal antibodies in the treatment of diseases such as cancer, rheumatoid arthritis, psoriasis, Crohn’s disease, systemic lupus erythematosus, cardiovascular disease, multiples sclerosis, ulcerative colitis, transplant rejection is called immunotherapy.
FDA regulations for the manufacture of monoclonal antibodies
The FDA(Food and Drug Administration agency) has designed a document called “ Points to Consider” that advises the manufacturers to consider the factors involved in the testing and production of monoclonal antibodies that are intended for human use and the identified information that is ought to be materialized in the Investigational New Drug or biologics license applications.
For monoclonal antibodies to be used as drug reagents the FDA has also published some guidelines that accentuate the performance characteristics of the reagent, biological safety, and the residual amounts of the reagent that is potentially present in the final product. The Centre for Biologics Evaluation and Research(CBER) and the Centre for Drug Evaluation and Research agencies regulate the applications of monoclonal antibodies.
Monoclonal antibody cocktail (Two different mAbs) injection imparts protection against SARS-CoV-2 by binding to different epitopes.
Recently, on January 24, 2022, a statement from Patrizia Cavazzoni M.D and Director(Center for Drug Evaluation and Research) was released which stated that the FDA revised the authorizations and use of two monoclonal antibody treatments–bamlanivimab and etesevimab(administered together) and REGEN-COV(casirivimab and imdevimab) due to the omicron variant(mAbs may not show desired efficacy) which intermittently keeps evolving.
Conclusion
Monoclonal antibodies are protein molecules that are biologically synthesized in the laboratory. In organic chemical reactions, a monoclonal antibody serves as a template and catalyst. They are also used as tools in the assessment of the structure and function of a molecule. They are also used to administer drugs to an individual suffering from cancer. Production of monoclonal antibodies is an expensive approach because even a slight change in the epitope of an antigen configuration can lead to undesirable effects; to be meticulous, this supervenes the curtailment of their application.
March 13, 2022