Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) is high-resolution gel electrophoresis that aids in the separation of complex proteins.
A gel viewed under UV light to analyse the bands
SDS PAGE technique is based on the addition of an anionic detergent to make the proteins negatively charged is associated with their molecular mass; after denaturing the proteins. Once the proteins are treated with SDS, the mixture of proteins is separated using a polyacrylamide gel matrix. When the current is provided, individual proteins from the protein mixture will migrate based on their molecular mass. Earlier, there was less requirement to separate proteins from a biological mixture. However, in recent years studies on proteins have become an important component concerning immunology, biochemical analysis, serological samples, etc. Therefore, SDS PAGE is an essential technique utilized to separate protein mixtures in addition to other biological samples such as DNA, RNA, and proteins.
Table of Contents
Principle
When the current is given in the gel electrophoresis, protein macromolecules present at the cathode will move towards the anode. This migration is based on the molecular mass of the macromolecules. All protein, DNA, and RNA are negatively charged macromolecules. Therefore, when the current is passed, the negatively charged molecules will migrate towards the positively charged anode. Moreover, the rate of migration is affected by the size of the molecules; molecular mass. Proteins with high molecular mass migrate slower than those with lower molecular mass that migrate faster. These migrated proteins can be observed as bands from the source (cathode) to the end (anode).
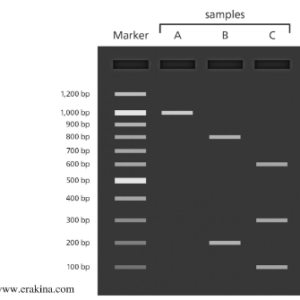
An illustration of bands viewed after electrophoresis
The separation of proteins using the SDS PAGE method involves a gel matrix made of polyacrylamide that assists in separating the proteins based on their structure. The migration of proteins is influenced by their charge and structure. During migration, the smaller macromolecules tend to migrate faster due to low resistance during the electrophoresis, while the larger molecules face higher resistance; hence they migrate slower. The purpose of using SDS PAGE is to terminate the influence of protein structure and its charge. Instead, these proteins are separated based on the length of their polypeptide chain.
SDS is an anionic detergent in addition to reducing agents introduced into the protein sample. The role of SDS is to denature the proteins by disrupting the disulfide bonds in proteins, thus breaking their tertiary structure. PAGE is a polyacrylamide gels function to provide more resistance to the larger protein molecules from traveling faster than smaller protein molecules. Since the SDS denatured proteins are dependent on the differences in their relative molecular mass of the polypeptide structure. A standard ladder of known protein mass is added to the gel and the samples. The known molecular mass can be used to plot a graph to estimate the unknown mass of the protein and the Rf (relative migration distance) of the unknown.
Working
Polyacrylamide gel is prepared to contain wells to add the protein samples and a standard ladder of known molecular mass of protein. These unknown protein samples are treated with SDS during the isolation stage in addition to dithiothreitol, a reducing agent to disrupt disulfide bonds to avoid any tertiary folding of the protein. After placing the gel in the electrophoresis buffer and loading the samples, the current is passed from the cathode (negatively charged) to an anode (positively charged). Ethidium bromide is commonly used for staining, after which the bands are then viewed under UV light for viewing.
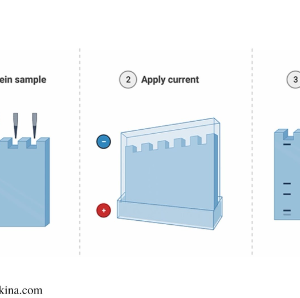
Gel electrophoresis shown in three stages
SDS PAGE is used to separate complex proteins by estimating their relative molecular mass and the concentration in the sample. Moreover, the purity of the sample protein can be analysed by other methodologies. Various staining techniques are utilised for different proteins to determine the biochemical properties of rare and novel proteins. Extraction of these proteins is obtained by employing special techniques such as peptide mapping, western blotting, and 2-D electrophoresis. These techniques are also used to determine gene products, segregate isoenzymes in proteins, and estimate similarities among the proteins.
Applications
The range of applications for the SDS PAGE technique is wide. Apart from using this technique for separating protein molecules, they are also used in recording the molecular mass of these molecules. This technique is also used to determine the purity of the sample protein and estimate the concentration of a protein in sample mixtures. Additionally, the structural and biochemical properties of the protein samples can be determined to identify the protein. This technique is utilised in forensics, biotechnology, the medical field, and other biochemistry research.
12/05/2022