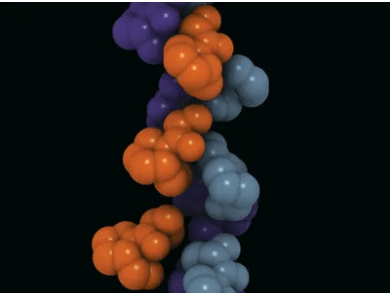
Collagen provides structural integrity to the endoskeleton and is the most abundantly found protein in the human body.
Collagen: An overview
Collagen is found in the extracellular matrix of all connective tissues in the human body and is, hence, the most abundant protein. It is composed of fibrils which are made up of amino acids bound together in the form of a triple helix. Collagen is found in the following connective tissues-
- Cartilage
- Bones
- Tendons
- Ligaments
- Skin
It is important to note that collagen fibres can be either rigid or compliant based on the degree of mineralization. In the case of cartilage, there is a gradient of mineralization leading to a structure that shows a certain degree of both rigidity and compliance.
Structural characteristics
Collagen is made up of units called tropocollagen molecules which further aggregate to form collagen fibrils. Some important structural characteristics of collagen tissues are as follows-
- A collagen fibril is approximately 1.5 nm in width and 300 nm in length.
- The tropocollagen molecules aggregate to form a left-handed helix with the amino acid Glycine towards the interior of the helix. This is so because Glycine is the smallest amino acid without a residual side chain that can be accommodated in the small pocket present towards the interior of the left-handed, tropocollagen helix.
- Multiple left-handed helices twist around each other to form a right-handed superhelix which is stabilised by hydrogen bonds.
- The repeated occurrence of Glycine in the left-handed tropocollagen helix provides stability and allows hydrogen bonding and intermolecular interactions in the form of cross-links.
- The arrangement of collagen fibrils is what makes each tissue different. For example, in bones (which are rigid), collagen fibrils are parallelly-arranged and tightly packed attributing to bone density and rigidity. Collagen fibrils are arranged in a multitude of concentrations and patterns.
Procollagen is the precursor to collagen fibrils and is composed of three component helical structures with the amino acids, glycine and proline as its main components.
Uses of collagen
Collagen has a multitude of uses and benefits, ranging from skincare to food and medicine. Some of the most significant uses of collagen are-
- Collagen is responsible for the elasticity of the skin and hence, upon its degradation, wrinkles and fine lines are observed. Collagen supplements are used to prevent ageing and are also used to improve the health of skin, hair and nails.
- Another cosmetic use of collagen is its use in cosmetic surgeries. Cosmetic surgeries involve tissue reconstruction and alteration. Due to its gradient of rigidity and compliance, collagen is highly suited for such surgeries and processes.
- Medical applications of collagen include- the prevention of ventricular fibrillation by the layering of collagen with smooth muscles at varying densities, the use of collagen in bone grafts, tissue regeneration and wound healing (ex: burns).
- When exposed to denaturing agents such as heat, collagen fibrils become partially uncoiled to form globular structures that are semi-solid. This denaturation of collagen describes the synthesis of gelatin which is widely used in the food industry.
- The skin and sinews of animals have been used for millennia as glue. This can be attributed to collagen, which, when denatured becomes sticky.
Collagen-associated disorders
Collagen-associated disorders are mainly caused due to genetic deformities and nutritional deficiencies. Some significant collagen-associated disorders are-
- Osteogenesis imperfecta- is caused due to mutations in the genes coding for Type I collagen. This disorder is characterised by weak bones and irregular connective tissues.
- Chondrodysplasias- is a skeletal disorder caused due to mutation in the genes coding for Type II collagen.
- Ehlers-Danlos syndrome- this disorder is of thirteen types with each type being attributed to a different mutation. Some of the rarer types can be lethal and even lead to arterial ruptures.
- Alport syndrome- is mainly a genetic disorder of the X-linked dominant type, however, it can also be passed down autosomally. Symptoms of this disorder include kidney and eye dysfunction accompanied by loss of hearing during early childhood.
- Knobloch syndrome- is caused due to mutation of one of the collagen-forming genes. Symptoms include protrusion of brain tissue and retinal degeneration. This disorder has a hereditary link.
Conclusion
Collagen fibrils are long structures made up, primarily, of amino acids. It is the most abundant protein in the body as it is present in all connective tissues. Collagen fibrils improve the structural integrity of the hair, skin and nails. Nutritional deficiencies can lead to the underproduction of collagen in the body resulting in weak bones and skin degradation. Collagen provides multiple medicinal benefits. It is also widely used in the food industry in the form of gelatin and also in the industrial sector as an adhesive. Mutations in the genes that code for collagen production can lead to several chronic illnesses.