What Is Ozone?
The reaction of UV rays with molecular oxygen forms ozone. The ozone layer is formed as a distinct layer in the lower stratosphere. By nature, a balance is maintained between the production and degradation of ozone in the stratosphere. The enhancement of ozone degradation by chlorofluorocarbons (CFCs) disrupts this balance.
Ozone Layer
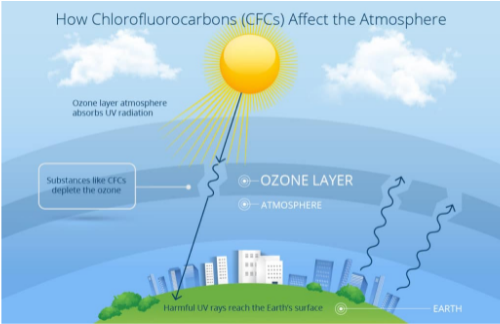
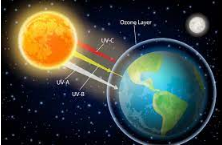
The ozone layer protects humans and other life forms from exposure to harmful short-wavelength ultraviolet (UV) radiation by acting like a fragile shield. When exposed to intense UV light in the stratosphere, some compounds, which are called ozone-depleting substances (ODSs), release chlorine or bromine, contributing to ozone depletion.
CFCs
ODSs that release chlorine includes CFCs, hydrochlorofluorocarbons, carbon tetrachloride, and methyl chloroform. CFCs are molecules made up of three oxygen atoms, O3, in Earth’s stratosphere. These could destroy the ozone. The stratospheric ozone absorbs ultraviolet radiation reaching the Earth’s surface.
These compounds are inert and essentially nontoxic, which are the characteristics that led CFCs to be used in applications such as refrigeration, air conditioning, and aerosol spray cans..The above-mentioned characteristics, however, also caused CFCs to be dangerous to life on Earth. The aerosols in the stratosphere create a surface on which the chlorine released from CFCs can destroy the ozone. CFCs were also used to manufacture Styrofoam. These were capable of breaking apart ozone molecules, rapidly causing the breakdown of ozone in the stratosphere.
Effect of CFCs
Upon reaching the atmosphere, CFCs move slowly toward the stratosphere. Subsequently, these compounds are broken down by UV radiation, thereby releasing chlorine atoms, which can destroy the ozone molecules. When a chlorine atom reacts with an ozone molecule, it absorbs one of the ozone molecules and three oxygen atoms, forming a molecule of chlorine monoxide. Subsequently, an ozone molecule is destroyed. Scientists discovered that one chlorine atom can destroy as many as a thousand ozone molecules. Ozone depletion has particularly occurred over the Antarctic region. It resulted in the formation of a thin ozone layer called an ozone hole. The ozone hole is a good example of excess pollution. The ozone layer above the Antarctic has been impacted by pollution since the mid-1980s.
During winter in the Antarctic, icy clouds form at very low temperatures because of air swirling in a vortex. These icy cloud particles react on the surface and release chlorine from chemical compounds such as CFCs, which in turn react with ozone. During spring, when sunlight returns, chlorine begins to destroy ozone. Shorter-wavelength UV radiations, when compared with UV-B, are completely absorbed by Earth’s atmosphere. UV-B damages the DNA, which may result in mutation, leading to skin aging and skin cancer. In the human eye, the cornea absorbs a high dose of UV-B radiation, which causes inflammation of the cornea, resulting in snow-blindness, cataracts, etc.
The Montreal Protocol
In recognition of the adverse effects of ozone depletion, an international treaty called the Montreal Protocol was signed in Montreal, Canada, in 1987. The protocol became effective in 1989. It is proposed to control the emission of ozone-depleting substances. Subsequently, many more efforts have been made and protocols have been introduced in developed and developing countries for the reduction of CFC emissions. The Montreal Protocol provides a framework for international control of emissions of CFCs, and its modification in 1990 led to the elimination of further production of CFCs within the next decade.
As currently observed in the Antarctic ozone hole, large ozone depletion occurs in the polar region annually, instead of being seasonal. A Very high decrease in temperature is observed in response to atmospheric changes and a decrease in shortwave radiation being absorbed by ozone. It is estimated that by 2053, in the tropical lower stratosphere, ozone levels will remain constant; by 2058, they will reach near zero because of heterogeneous chemical processes being observed in the ozone hole in the Antarctic.
CFC refrigerants, such as the once popular R12, have the highest ozone-depleting rating and also lead to greenhouse gasses. The greenhouse gas effect, as the name suggests, relates to the warming of the Earth’s surface temperatures. This distorts the balance in the Earth’s ecosystems.
The ozone levels in the stratosphere have been recovering at rates of 1-3 percent since 2000. It is estimated that the Northern Hemisphere and mid-latitude ozone will be fully recovered in the 2030s, while the Southern Hemisphere ozone will recover in the 2050s, and the polar regions will recover before 2060.
Overcoming the CFC effect
Currently, substitutes for CFCs are being developed rapidly, by focusing attention on hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs). These compounds can be primarily removed through oxidation in the lower atmosphere. The Montreal Protocol has banned the use and production of CFC refrigerant in all the countries that have implemented the protocol.