Introduction
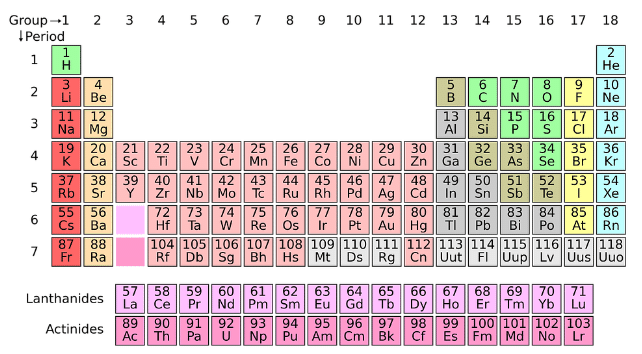
All the 118 elements which have been decided and organized in the periodic table may be notably labelled into 3 groups: metals, non-metals, and metalloids. Metals are electropositive factors which can donate electrons, even as non-metals are electronegative factors which can preserve electrons. Metalloids are the 0.33 beauty whose homes are intermediate of metals and non-metals, for example, silicon.
What is Non-Metal?
Non-metals are the factors which shape bad ions with the aid of the usage of accepting or gaining electrons. Non-metals usually have 4, 5, 6 or 7 electrons in their ultimate orbit which forces them to accept electrons to complete their octet.
Non-metals are the ones which lack all of the metal attributes. They are particular insulators of warmth and power. They are typically gases and now and then liquid. Some are even sturdy at room temperatures like Carbon, sulphur and phosphorus.
Characteristic homes of non-metals are excessive ionization energies and excessive electronegativity. Owing to one’s homes, non-metals usually gain electrons even as they react with unique compounds, forming covalent bonds. Among the non-metals, the anionic dopants have a robust impact on the VB. Non-Metal dopants consist of carbon, nitrogen, Sulphur and iodine.
The following are the overall homes of non-metals
- The atoms of non-metals will be inclined to be smaller than the ones of metals. Several of the opportunity homes of non-metals result from their atomic sizes.
- Non-metals display very low electric powered conductivities. Low or non-existent electric powered conductivity is the maximum important belonging that distinguishes non-metals from metals.
- Non-metals have excessive electronegativities. This method ensures that the atoms of non-metals have a robust tendency to draw greater electrons than what they may commonly have.
- Under everyday situations of temperature and pressure, a few non-metals are decided as gases, a few are decided as solids and one is decided as a liquid. In contrast, besides mercury, all metals are solids at room temperature. The reality is that some non-metals exist as drinks or gases that non-metals typically have highly low melting and boiling factors under everyday atmospheric situations.
- In their sturdy state, non-metals will be inclined to be brittle. Therefore, they lack the malleability and ductility exhibited with the aid of the usage of metals.
Physical Properties of Non-Metallic factors
- Ductility is the assets of the fabric to be stretched into wires; however, non-metals aren’t ductile besides carbon, as carbon fibres locate and are used in a sizable style of industries in the aspect of sports activities and track equipment.
- Another asset feature of metals is absent in non-metals known as malleability. They can’t be drawn into sheets as they will be brittle and damage the usage of pressure.
- They aren’t lustrous as they do not have any colourful appearance.
- They aren’t sonorous and no longer produce a deep ringing sound even as they will be hit with some distinct materials. They also are awful conductors of warmth and power besides graphite.
Chemical Properties of Non-Metals
- Reaction with Water
A non-metal does not react with water, however, it’s also very reactive in air, this is why a number of them are saved in water. For example, one of the especially reactive non-metals is phosphorus and it catches hearthplace even as uncovered to air this is why it’s far saved in water to save you its touch with atmospheric oxygen.
- Reaction with Acids
None of the non-metals is perceived to react with acids.
- Reaction with Bases
The response among non-metals and bases is a completely complicated one. The response of chlorine with bases like sodium hydroxide offers merchandise like sodium hypochlorite, and sodium chloride similarly to water.
- Reaction with Oxygen
Oxides of non-metals are standard even as it reacts with oxygen. The oxides of non-metals are either acidic or neutral but can never be basic.
When sulphur reacts with oxygen, it turns into sulphur dioxide.
S + O2 → SO2
When sulphur dioxide reacts with water it works as sulphurous acid.
SO2 + H2O → H2SO3
Significance of Non-Metals
- For the steering of ammonia, nitric acid and fertilizers, nitrogen is used.
- Chlorine is used to purify water.
- Hydrogen can be very beneficial as rocket fuel.
- Carbon may be used to make pencils even as it’s far in graphite shape.
- Sulphuric acid is ready for the usage of sulphur.