Introduction
The earth is a huge mass structure that is created by the big bang that happened in the galaxy. The big bang didn’t only create earth, it also gave us many resources. The earth’s crust consists of minerals and metals which are used in our daily life and for the development of the country. Today we are going to discuss metals and the physical and chemical properties they possess.
Metal
Metal is an element with a common property under which many elements come under it. A metal is a thing that is strong, shiny, has a high melting point, ductility, conductivity, etc. An element that has these characteristics in nature is considered a metal. The surrounding around us is made up of things that are metal and non-metals. To classify this, scientists found the periodic table.
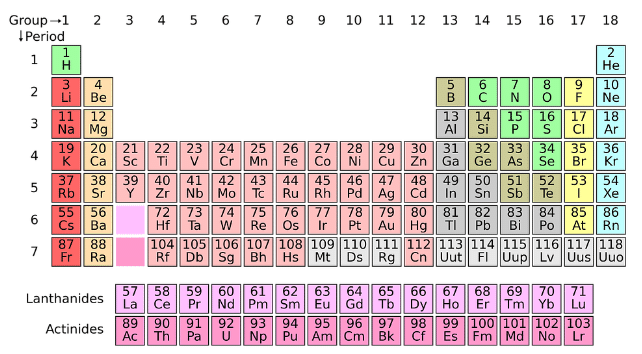
A periodic table consists of elements that are found on earth and separated as metal and non-metal. Today’s modern periodic table consists of 118 elements among which 18 are non-metals, 7 are metalloids and 93 are metals. The row of the table is called period and the columns are called groups. By using this, people are able to find which element is metal and which is not. There are various types of metals on earth. They are:
- Alkaline earth metals
- Alkali metals
- Transition metals
- Actinides and
- Lanthanides
Every element present on earth will be in the periodic table and even if a new element is found it will be added to this list. The periodic table separates metals and non-metals on each side of the table.
Physical properties and uses
Metal has its own characteristics and uses. The physical properties of metals are:.
- Metal is normally present in a solid-state but some metals can also be in a liquid state. For example, Mercury is a metal that is in a liquid state.
- Metals are shiny and rigid in nature. That’s why they are used in making jewelry and in decoration materials.
- Silver reflects up to 90 percent of light. So they are used in the coating of mirrors because of their impeccable reflecting property.
- Many metals are hard in structure but some of the metals like sodium, potassium, and magnesium are easy to cut. They can be cut with the help of knives.
- Metals are able to be hammered to change shapes. This is called malleability. Through this metals can be hammered into little sheets, shapes, etc. to make different products. For example, Galvanized steel sheet, aluminum sheet, etc.
- Copper is a metal that is obtained from the earth’s crust and is useful in making many products. With their high conductivity to electricity, they are made into wires and used to pass electricity in homes.
- Gold is a luxury metal used in making ornaments. But it is also used in making circuit boards on a computer.
- Cooking utensils are made of stainless steel and copper for their high conductivity of heat.
- Many metals are sonorous in nature. Through this people created bells for churches and bicycles.
- With the hard rigidity structure metals like iron are used in the construction of buildings, railway tracks, and used in automobiles.
- With their high melting point, the metals can be melted and molten into any shape.
The above are some of the physical properties and uses of metals.
Chemical properties
- When a metal is burned, they combine with oxygen in the surrounding and form metallic oxides. Eg: Magnesium burned with oxygen can be changed into magnesium Oxide. This metal oxide can turn a red litmus paper blue. This change can bring many changes in a chemical reaction.
- Many metals can react with water in a chemical reaction and change into hydroxides. Eg: Sodium can react vigorously with water and forms sodium hydroxide and hydrogen.
- Metals easily corrode to strong chemical reactions.
- Some metals start rusting when they get into contact with water for a long time.
- Many metals are used in the process of electrolysis.
We come to the end of the blog in which we see what metals are and their physical and chemical properties they possess. Visit Erakina website for more topics to learn.