Introduction Bases
Chemistry is a subject of reaction, equation, volumetric analysis, solubility test, etc. They explain to us the source of the subject and the nature of metals and their significance. Let’s see what base and the types of bases.
What is Base?
Types of bases
A Base is a property that neutralizes an acid with the help of hydrogen ions. A base is mostly in a mineral form that acts with acid and can form water and salt. Bases are mostly oxides, and carbonates in metals. A base can be identified by its routine of donating an electron, accepting protons, or releasing hydroxide ions in an aqueous solution. Another way of finding a base is that they are slippery in touch, bitter in taste, and catalyze some reactions.
A base can be soluble in nature. The soluble bases are called alkali. Some of the hydroxides are alkali in nature. All alkali is base. But not all bases are alkali.
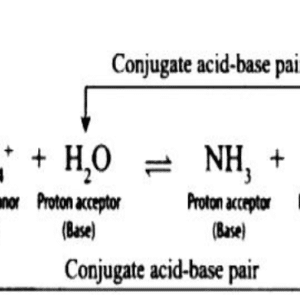
Base and Conjugal acid equation
A Base show many properties such as:
- A base solution can dissociate into ions to produce electricity.
- The pH value of the Base is more than 7.
- The litmus paper indicates blue in color when it gets into contact with a base.
- They taste bitter. It is advised not to taste them for the good of human health.
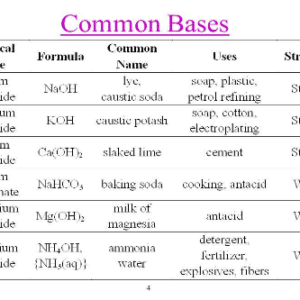
- Some of the bases are used as electrolytes which are sodium hydroxide and potassium hydroxide.
- The process of acid and base reacting together is called neutralization.
Types of Bases
Bases can be differentiated by their dissociation of ions in an aqueous solution. The type of bases are:
- A strong base is a property that dissociates ions in water which are able to remove a proton from a weak acid. Or, they are able to dissociate into ions completely in the solution. The strong bases are Sodium hydroxide and potassium hydroxide.
- A weak base is where the ions dissociate very less in water and contain a weak base and conjugate acid.
- A super base is way stronger and dissociates better than a strong base. It is because of the presence of alkali metals in the aqueous solution. A super base cannot stay longer in an aqueous solution because it is strongly basic in nature. The superior super base is ortho-diethynylbenzene dianion(C6H4(C2)2)2−.
- A neutral base is a base that forms a bond with the weaker acid and they both share the same electron pair which is from the base.
- A solid base will be active only when they are in a solid state.
These are some of the types of bases differentiated by their characteristic in nature.
Uses of Base
Base products
The base property of a substance is used in our daily life for several purposes. Let’s see some of the uses of the base.
- Some of the base properties are used to neutralize acids in reactions and industrial purposes.
- An antacid is made up of bases that help to balance the acid reaction and helps us with indigestion and heartburn.
- Not only does the base neutralize the acid in the reaction, it also helps in chemical reactions.
- It is used in making soaps.
- Strong bases like Arrhenius bases dissociate well in aqueous solutions. By this property, they are used in making products like laundry detergent, drain cleaner, baking soda, etc.
- A weak Bronsted Lowry base doesn’t completely dissociate in an aqueous solution and accepts a proton from other molecules. It is useful in making products like chewing gum, plastic, Alkaline batteries, ammonia, etc.
- Lewis base which is also called super base is used in making alcohol, hair dye, plaster, pesticides, Epsom salts, etc.
These are some of the products that are made from the base that we use in our daily life.
Acid and base are always opposite to each other but some species possess the feature of being either an acid or a base.
internal links:
Health Benefits Of Oatmeal – Erakina
Do you want to know health benefits recipe of Sambaram: spiced buttermilk
Mukesh Kumar U M
22/04/2022